Isi ihe dị iche n'etiti nitrate na nitrite bụ na nitrate nwere atọ oxygen atọ jikọtara na nitrogen atom ebe nitrite nwere atom oxygen abụọ jikọtara na atom nitrogen.
Ma nitrate na nitrite bụ anions inorganic nke nwere nitrogen na ikuku oxygen.Ma anions ndị a nwere ụgwọ eletrik -1.Ha tumadi ime dị ka anion nke nnu ogige.Enwere ụfọdụ ọdịiche dị n'etiti nitrate na nitrite;anyị ga-atụle ọdịiche ndị ahụ n'isiokwu a.
Kedu ihe bụ Nitrate?
Nitrate bụ anion inorganic nwere usoro kemịkalụ NO3-.Ọ bụ anion polyatomic nke nwere 4 atọm;otu nitrogen atọm na atọ oxygen atọ.The anion nwere -1 mkpokọta ụgwọ.Oke molar nke anion a bụ 62 g / mol.Ọzọkwa, anion a sitere na conjugate acid ya;nitric acid ma ọ bụ HNO3.N'ikwu ya n'ụzọ ọzọ, nitrate bụ njikọ njikọ nke nitric acid.
Na nkenke, nitrate ion nwere otu atom nitrogen dị n'etiti nke jikọtara ya na atọ oxygen atọ site na njikọ kemịkalụ covalent.Mgbe ị na-atụle usoro kemịkalụ nke anion a, ọ nwere njikọ atọ yiri nke NO (dị ka usoro resonance nke anion si dị).N'ihi ya, geometry nke molekul bụ trigonal planar.Atọm oxygen ọ bụla na-ebu ụgwọ - 2⁄3, nke na-enye ụgwọ zuru ezu nke anion dị ka -1.
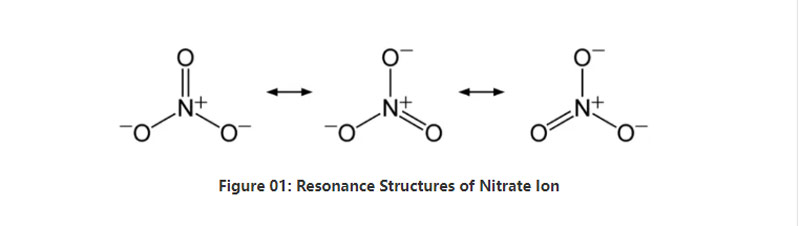
Na nrụgide ọkọlọtọ na okpomọkụ, ihe fọrọ nke nta ka ọ bụrụ ogige nnu niile nwere anion a na-agbaze na mmiri.Anyị nwere ike ịhụ nnu nitrate sitere n'okike n'ụwa dị ka nkwụnye ego;ntinye nitratine.Ọ kachasị nwere sodium nitrate.Ọzọkwa, nje nje nitrifying nwere ike imepụta ion nitrate.Otu n'ime isi eji nnu nitrate eme ihe bụ imepụta fatịlaịza.Ọzọkwa, ọ bara uru dị ka onye na-ahụ maka oxidizing na ihe mgbawa.
Kedu ihe bụ Nitrite?
Nitrite bụ nnu inorganic nwere usoro kemịkalụ NO2-.Anion a bụ anion symmetrical, o nwekwara otu nitrogen atom nke jikọtara ya na atọm oxygen abụọ nwere njikọ abụọ yiri NO covalent chemical bond.N'ihi ya, nitrogen atom dị n'etiti molekul.The anion nwere -1 mkpokọta ụgwọ.
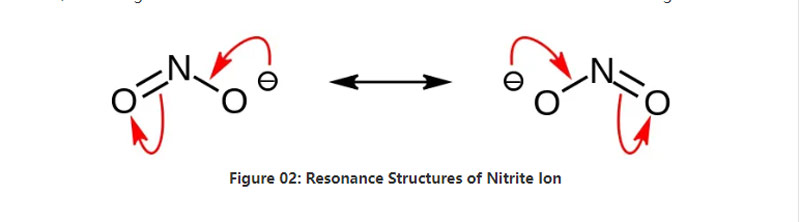
Oke molar nke anion bụ 46.01 g / mol.Ọzọkwa, anion a sitere na nitrous acid ma ọ bụ HNO2.N'ihi ya, ọ bụ conjugate isi nke nitrous acid.Ya mere, anyị nwere ike ịmepụta nnu nitrite na ụlọ ọrụ mmepụta ihe site na ịfefe uzuzu nitrous n'ime ngwọta sodium hydroxide.Ọzọkwa, nke a na-emepụta sodium nitrite nke anyị nwere ike ime ka ọ dị ọcha site na recrystallization.Ọzọkwa, nnu nitrite dị ka sodium nitrite bara uru na ichekwa nri n'ihi na ọ nwere ike igbochi nri site na uto microbial.
Kedu ihe dị iche n'etiti nitrate na nitrite?
Nitrate bụ anion inorganic nke nwere usoro kemịkalụ NO3- ebe nitrite bụ nnu inorganic nwere usoro kemịkalụ NO2-.Ya mere, isi ihe dị iche n'etiti nitrate na nitrite dabere na ihe mejupụtara kemịkalụ nke anions abụọ ahụ.Ya bụ;Isi ihe dị iche n'etiti nitrate na nitrite bụ na nitrate nwere atọ oxygen atọ jikọtara na nitrogen atom ebe nitrite nwere atom oxygen abụọ jikọtara na atom nitrogen.Ọzọkwa, ion nitrate sitere na conjugate acid ya;nitric acid, ebe ion nitrite sitere na nitrous acid.Dị ka ihe ọzọ dị mkpa dị iche n'etiti nitrate na nitrite ion, anyị nwere ike ikwu na nitrate bụ oxidizing gị n'ụlọnga n'ihi na ọ nwere ike na-enweta nanị Mbelata ebe nitrite nwere ike ime dị ka ma oxidizing na mbenata gị n'ụlọnga.
Oge nzipu: Mee-16-2022





